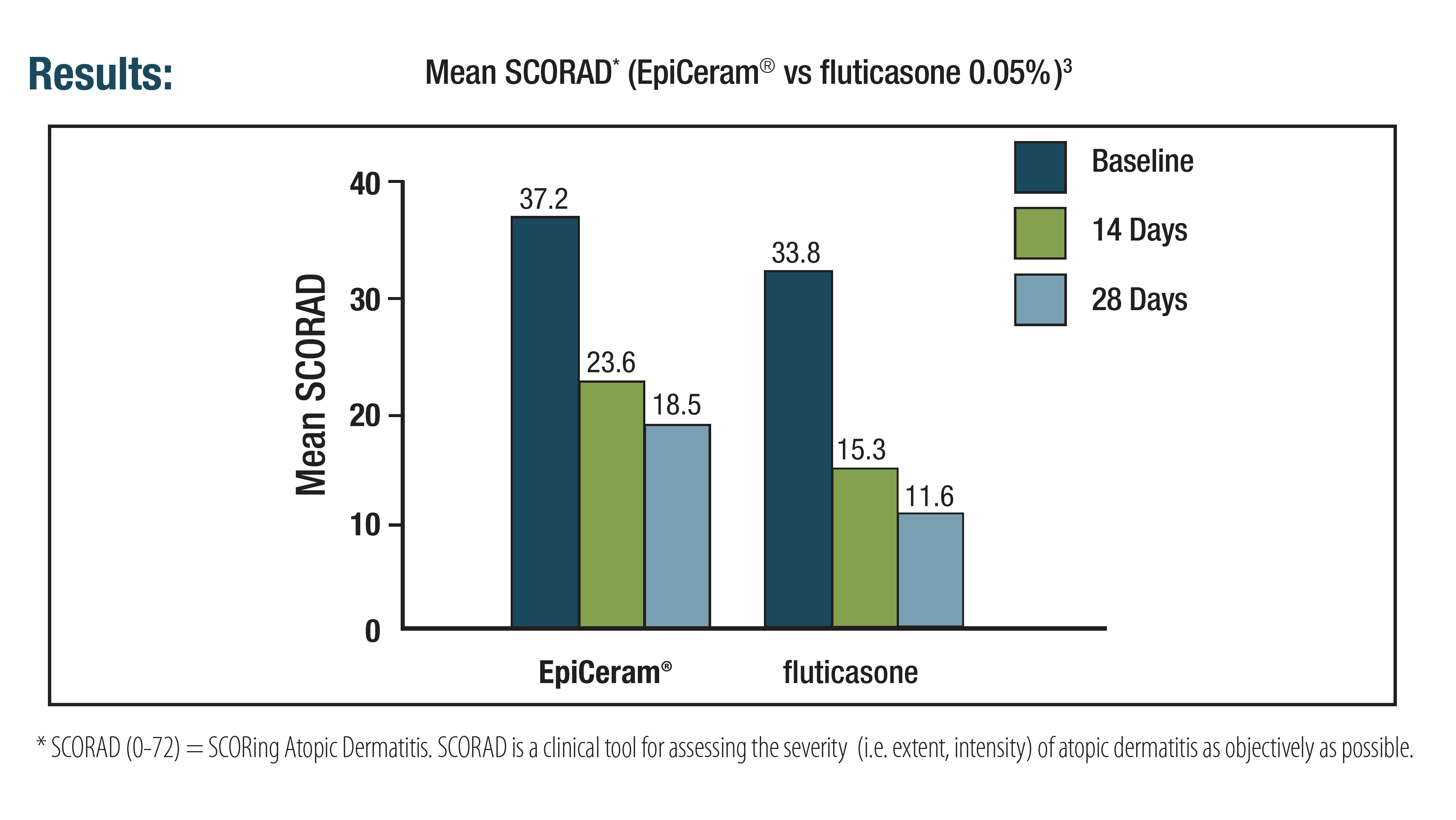
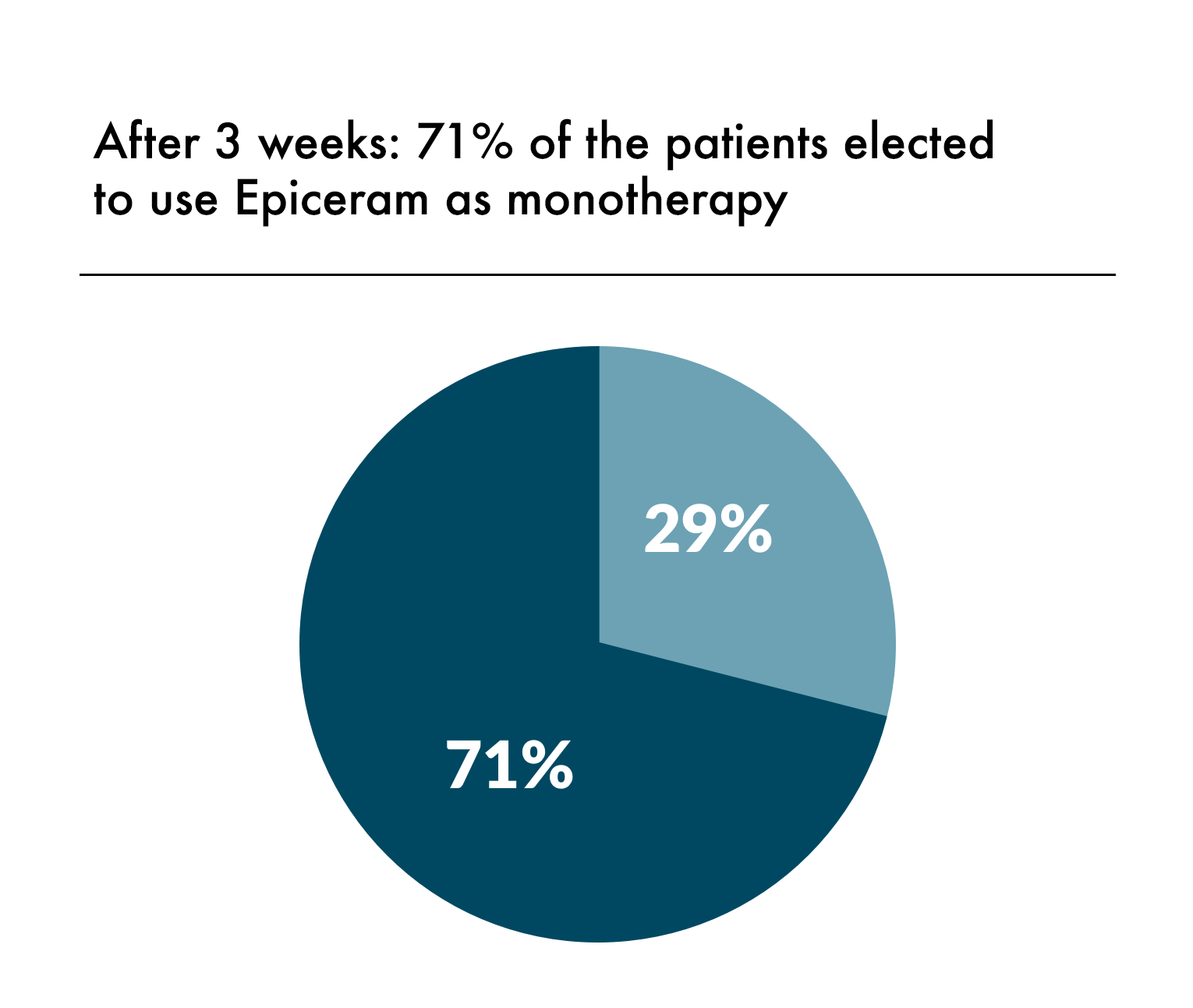
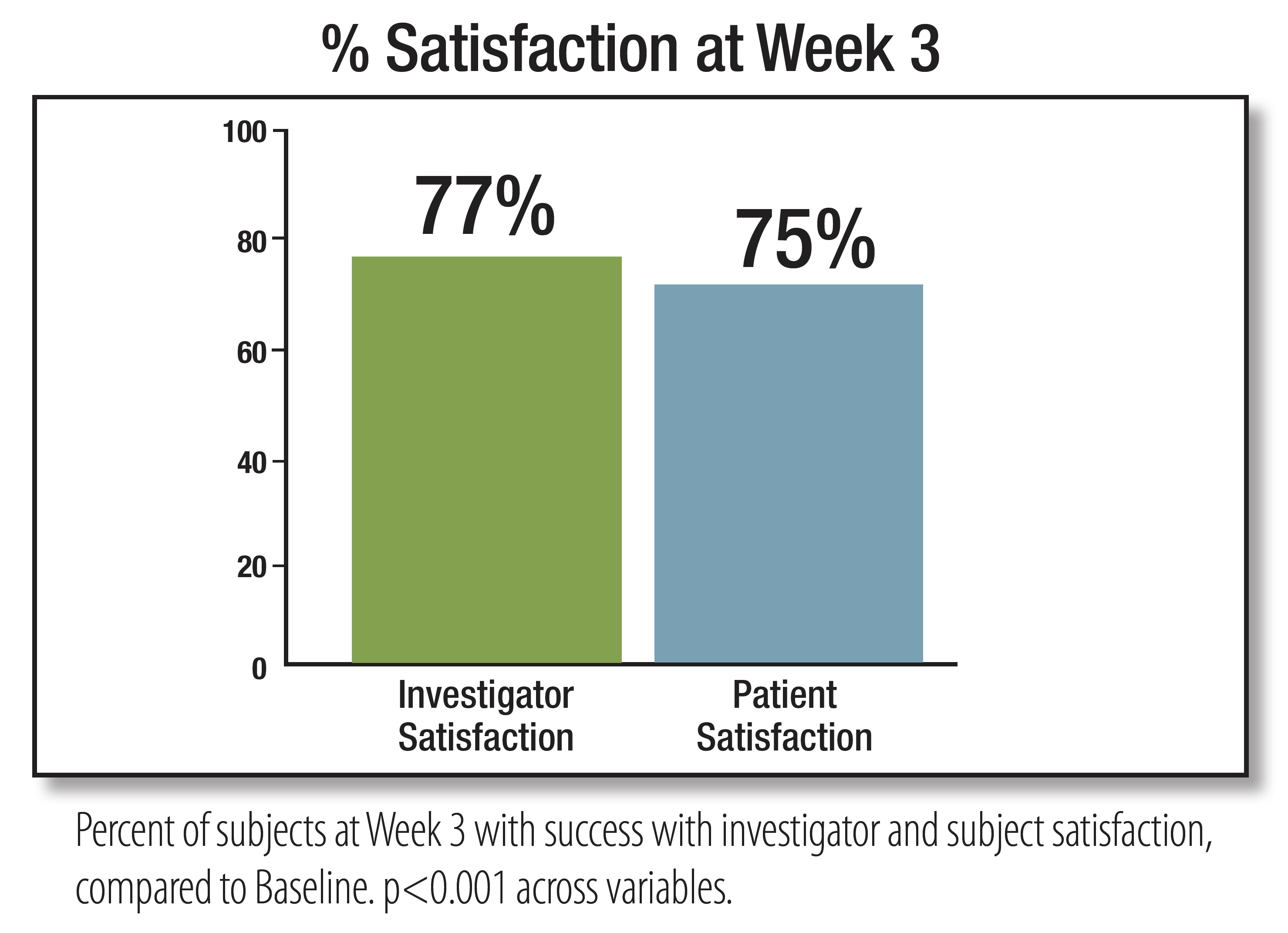
EpiCeram Helps Maintain a Healthy Skin Barrier That Goes Beyond Moisturizing
EpiCeram helps repair and heal the skin barrier through a unique delivery system not found in other products. EpiCeram contains 3 essential lipids– ceramides, conjugated linoleic acid (CLA), and cholesterol — in a physiologically balanced patented 3:1:1 ratio, which are reduced in patients with eczema and atopic dermatitis. EpiCeram is steroid-free, fragrance-free, noncomedogenic, paraben-free, and propylene glycol-free.
Help to repair the Skin Barrier with EpiCeram
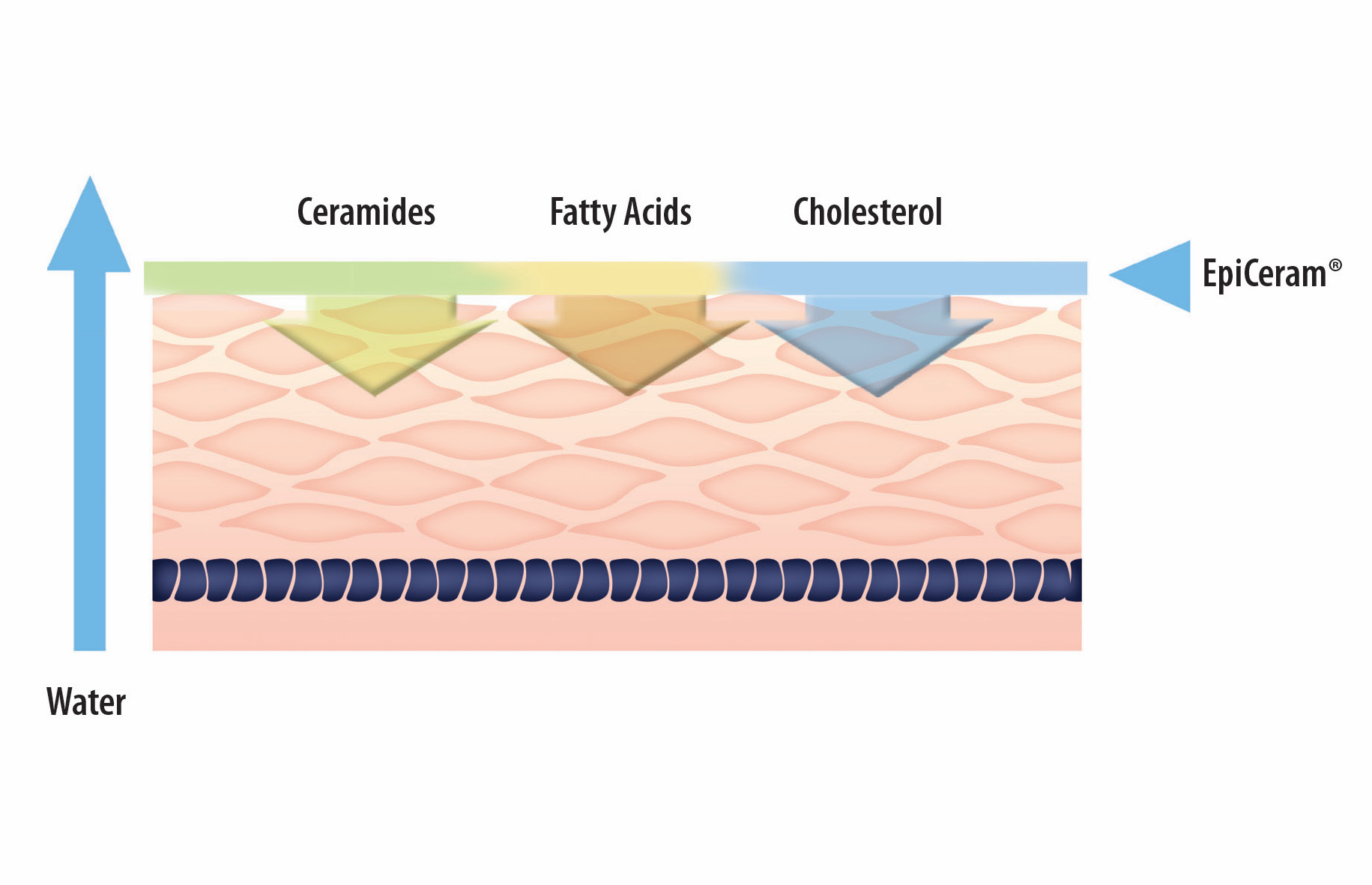
- Only topical treatment for atopic dermatitis formulated with the three essential physiological lipids in a patented 3:1:1 ratio ceramide dominant with unmatched lipid levels - 5% by weight with 3% ceramides
- Only EpiCeram utilizes the patented MultiSal™ release technology—a dual encapsulation system that allows for sustained delivery and enhanced absorption
- Steroid-free, fragrance-free, paraben-free and propylene glycol-free
- Well established safety profile, with no restrictions on chronic use or patient age
-
Can be used on any area of the body, including face and intertriginous areas
Reference: Data on File. Primus Pharmaceuticals, Inc.
Epiceram® [package insert]. Scottsdale, AZ Primus Pharmaceuticals Inc; 2018.
EpiCeram with Patented MultiSal™ Technology Allows for High-Lipid Deposition and Absorption Over Time
The MultiSal Encapsulation Delivery Technology is based on a unique proprietary system consisting of multi-component microspheres that contain submicron spheres. These submicron spheres are infused with the physiological lipid in an active form. Rubbing the emulsion onto the skin triggers this two-step delivery system. Once the microsphere is broken, the submicron spheres gradually deliver the physiological lipids over time. The special microsphere structure stabilizes the lipids from premature oxidation.
Real-World Cases
Before & After Treatment with EpiCeram
Adult Patient (Age 58, Female)
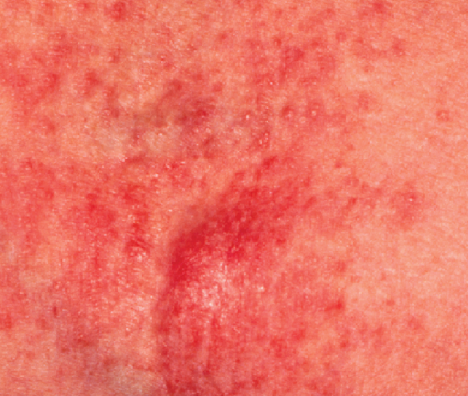
Before
Dx: Atopic dermatitis, dry skin, eczema
Rx: EpiCeram applied BID to all affected areas
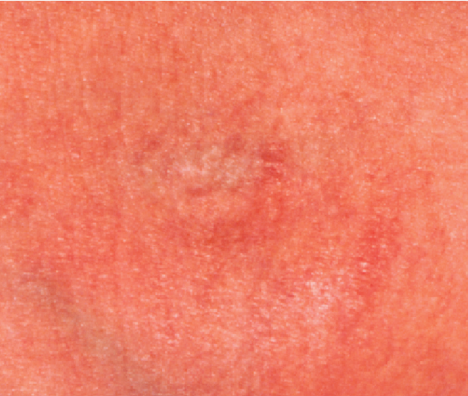
After 2 Weeks
90% reduction in the erythema, dryness, and scaling of the antecubital fossa bilaterally
Child Patient (Age 14, Male)
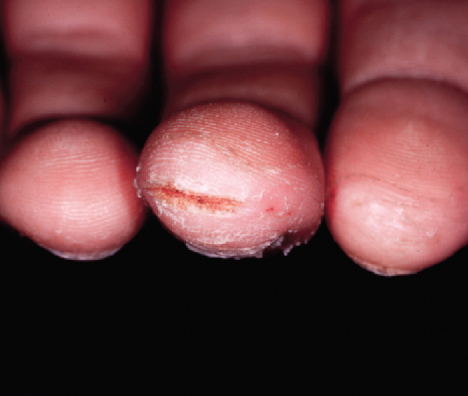
Before
Painful fissures located on distal tip of the left thumb, as well as the right middle finger
EpiCeram applied BID for 2 weeks
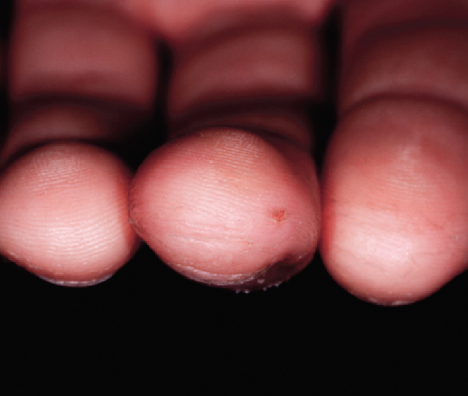
After 2 Weeks
Cracked, fissuring fingertips showed dramatic improvement in 48 hours
Completely pain and fissure free at this time
Adolescent Patient (Age 17, Male)
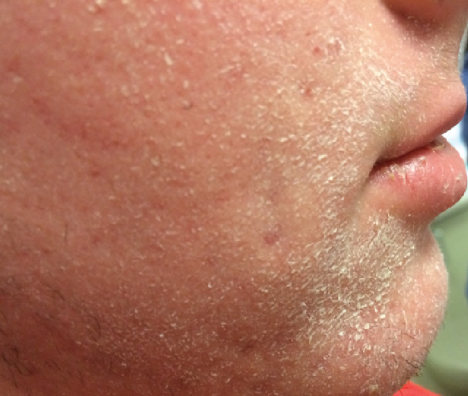
Before
Severe Xerosis Secondary to Isotretinoin Use
- Used many OTC moisturizers TID with worsening xerosis
Rx: EpiCeram minimum BID and can apply as needed throughout the day
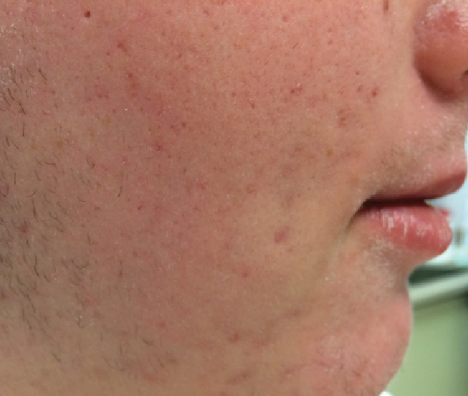
After 4 Weeks
Xerosis fully resolved
Continue to apply EpiCeram as needed throughout the day
Continue with normal cleansing routine
1. Bikowski J. Understanding the Structure, Function, and Strategies for Repair of the Epidermal Barrier. Practical Dermatology. 2009; May: 17-18
2. Data on File. Primus Pharmaceuticals, Inc.
3. EpiCeram [package insert]. Scottsdale, AZ. Primus Pharmaceuticals Inc; 2018.